Always a leader. Always dependable.
Grifols has provided more than 65 years of consistent supply and product support.
HyperTET is a tetanus immune globulin (TIG) that provides rapid immune coverage, indicated for prophylaxis against tetanus following injury in patients whose immunization is incomplete or uncertain. It is also indicated, although evidence of effectiveness is limited, in the regimen of treatment of active cases of tetanus. HyperTET is the only TIG currently available in the United States.
Although vaccines can provide lifelong protection, they can take weeks to build efficacy. A TIG provides immediate protection, which allows the vaccine the time needed to establish active immunity in high-risk situations.2,3 HyperTET contains high titers of tetanus antibodies and provides passive immunity for postexposure prophylaxis to a tetanus exposure.1
According to the Centers for Disease Control and Prevention (CDC), the use of a TIG such as HyperTET may, if promptly administered, reduce the potentially life-threatening risk of tetanus when administered concomitantly with the tetanus vaccine.1,4
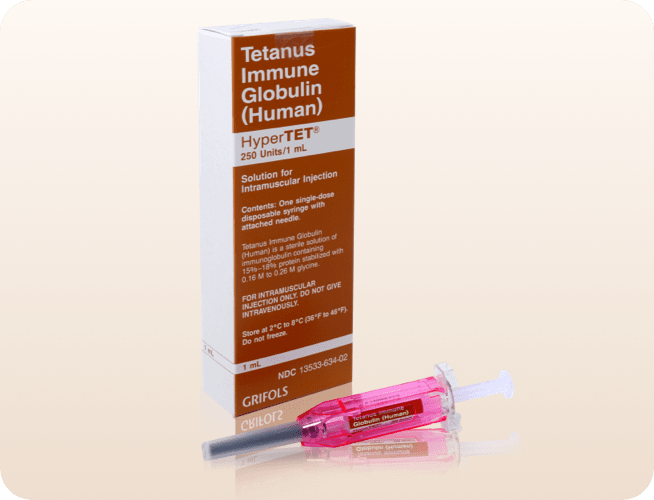
*BD UltraSafe® Needle Guard is a registered trademark of Becton, Dickinson and Company.
HyperTET supplies passive immunity to those individuals who have low or no immunity to the toxin produced by the tetanus organism, Clostridium tetani. The antibodies act to neutralize the free form of the powerful exotoxin produced by this bacterium.
If passive immunization is needed, the human tetanus immune globulin HyperTET, the only TIG available in the United States, is indicated.
HyperTET contains high titers of tetanus antibodies for PEP, providing rapid immune protection for up to 21 days. Peak blood levels of immunoglobulin G (IgG) are obtained approximately 2 days after intramuscular injection. The half-life of IgG in the circulation of individuals with normal IgG levels is approximately 23 days.
Twenty patients were treated with HyperTET (single doses of 3000 to 6000 international units) in combination with other clinical and nursing procedures on admission to the hospital or as soon as the diagnosis was made. Larger doses were given to patients with either a rapidly progressing illness or short incubation period.
Prior to this study, the reported national death rate for tetanus was 60%.
Grifols has provided more than 65 years of consistent supply and product support.
HyperTET is produced using a sophisticated caprylate/chromatography purification process that employs the highest quality and safety standards.
Main steps of the manufacturing process:
The capacity of the HyperTET manufacturing process to remove and/or inactivate viruses has been demonstrated by laboratory spiking studies on a scaled-down process model using a wide range of viruses with diverse physicochemical properties.
The caprylate/chromatography manufacturing process was also investigated for its capacity to decrease the infectivity of an experimental agent of transmissible spongiform encephalopathy (TSE), considered as a model for the variant Creutzfeldt-Jakob disease (vCJD) and Creutzfeldt-Jakob disease (CJD) agents. These studies provide reasonable assurance that low levels of vCJD/CJD agent infectivity, if present in the starting material, would be removed by the caprylate/chromatography manufacturing process.

HyperTET is made from human blood and may carry a risk of transmitting infectious agents, eg, viruses, the vCJD agent, and, theoretically, the CJD agent.
Grifols employs a comprehensive tracking system called PediGri® that ensures full traceability from every donation.
4.0 units/kg*
250-IU IM injection
| Routine Prophylaxis Dosage Schedule | Dosage |
|---|---|
|
Children younger than 7 years of age |
4.0 units/kg* |
|
Children 7 years of age and older and adults |
250-IU IM injection |
Standard therapy for the treatment of active tetanus including the use of HyperTET must be implemented immediately. The dosage should be adjusted according to the severity of the infection
| Active Tetanus | Dosage (under 250-IU IM) |
|---|---|
|
All ages |
Standard therapy for the treatment of active tetanus including the use of HyperTET must be implemented immediately. The dosage should be adjusted according to the severity of the infection |
J1670
Injection, tetanus immune globulin, human, up to 250 units
96372
Therapeutic, prophylactic, or diagnostic injection (specify substance or drug); intramuscular
A35
Other tetanus
Z23
Encounter for immunization
Z41.8
Encounter for other procedures for purposes other than remedying health state
13533-0634-02
250-unit syringe
| Coding System | Coding for HyperTET | Description |
|---|---|---|
|
HCPCS (Healthcare Common Procedure Coding System) Code* |
J1670 |
Injection, tetanus immune globulin, human, up to 250 units |
|
Administration Procedures CPT Code† |
96372 |
Therapeutic, prophylactic, or diagnostic injection (specify substance or drug); intramuscular |
|
ICD-10-CM‡ |
A35 |
Other tetanus |
|
Z23 |
Encounter for immunization |
|
|
Z41.8 |
Encounter for other procedures for purposes other than remedying health state |
|
|
Product NDC§ |
13533-0634-02 |
250-unit syringe |
Submit a request to get in contact with a representative
Important Safety Information for HyperTET® (tetanus immune globulin [human])
HyperTET® (tetanus immune globulin [human]) is indicated for prophylaxis against tetanus following injury in patients whose immunization is incomplete or uncertain.
HyperTET should be given with caution to patients with a history of prior systemic allergic reactions following the administration of human immunoglobulin preparations.
In patients who have severe thrombocytopenia or any coagulation disorder that would contraindicate intramuscular injections, HyperTET should be given only if the expected benefits outweigh the risks.
Slight soreness at the site of injection and slight temperature elevation may be noted at times. Sensitization to repeated injections of human immunoglobulin is extremely rare. In the course of routine injections of large numbers of persons with immunoglobulin, there have been a few isolated occurrences of angioneurotic edema, nephrotic syndrome, and anaphylactic shock after injection. Administration of live virus vaccines (eg, MMR) should be deferred for approximately 3 months after tetanus immune globulin (human) administration.
HyperTET is made from human plasma. Products made from human plasma may contain infectious agents, such as viruses and theoretically, the Creutzfeldt-Jakob disease (CJD) agent that can cause disease. There is also the possibility that unknown infectious agents may be present in such products.
Please see full Prescribing Information for HyperTET.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
References: